Aspiration and acute lung injury in organophosphorus insecticide poisoning
Introduction
The weaponised organophosphorus (OP) compound sarin was recently used in Damascus, Syria (Jul 2013) to devastating effect (1). Yet, the most common form of OP poisoning is actually from insecticide ingestion. WHO believes that insecticide poisoning is the main cause of suicide globally (2) resulting in 200,000 annual deaths.(3) Poisoning with OP insecticide has central and peripheral cholinergic effects, resulting in an unconscious paralysed patient with respiratory failure. Approximately one third of patients are intubated, but of these up to 50% die.(4,5) Our hypothesis was that pulmonary aspiration of ingested insecticide, its solvent and surfactants, or gastric contents may be causing acute lung injury and contributing to this statistic.(6)
Minipig aspiration model
Our research team in Edinburgh has used a Gottingen minipig toxicology model for the last 5 years to investigate insecticide poisoning. Previous work has shown that ingestion of 2.5mls/kg of the OP pesticide dimethoate emulsified concentrate 40% (EC40) alone causes respiratory arrest in 30 mins (6) and a mild lung injury- shown by bronchoalveolar lavage (BAL) analysis (unpublished).
To explore the above hypothesis, a 48 hour pulmonary aspiration study (n=26) was designed to investigate the mechanism and severity of lung injury created through pulmonary instillation of 0.5ml/kg mixtures of porcine gastric juice (GJ), OP, and/or its solvent cyclohexanone. These intervention groups were compared to both saline control and sham bronchoscopy i.e. placement of a bronchoscope in the right lung and immediate removal without instillation of any intervention mixture.
The model allowed unique observation of both a direct and indirect lung injury through use of a bronchial blocker in the left lung whilst the intervention mixture was administered through a sheathed bronchoscope and epidural catheter in the right lower lung lobe.
Animals
The study was approved by the Institutional Ethical Review Committee and was licensed under the Animal (Scientific Procedures) Act 1986. Following shipment from Ellegaard Denmark, the 20 Göttingen minipig sows were acclimatized for at least 1 week in laboratory housing and maintained according to Ellegaard Göttingen minipig (7,8) and Home Office (UK) guidelines. The mean (range) body mass of the female minipigs was 28.0 (23.5 - 31.5) kg and they ranged in age from 9 - 14 months. The animals were housed in groups of 2 - 6 where ad libitum water was available. Health checks were conducted daily (at feeding time) and a veterinary inspection was conducted at least once a week. The animals were not fasted and had constant access to straw before being studied.
Results
Aspiration of OP and GJ causes pulmonary neutrophil sequestration, alveolar haemorrhage, interstitial oedema, and disruption of the alveolar-capillary membrane (figure 1).
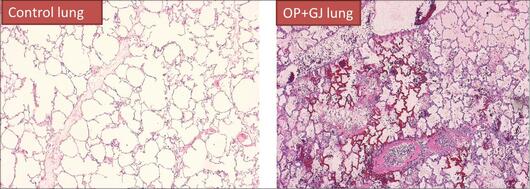
Figure 1: Pulmonary histopathology of a sham control pig (left) and a lung directly exposed to OP+GJ (right) 48 hours later. The OP+GJ lung shows pulmonary oedema (pink hue in the alveoli), neutrophil collections in the alveoli and interstitium (blue dots), necrotic areas of alveoli with widespread haemorrhage. Pictures were created from, formalin fixed, haematoxylin and eosin stained lung tissue at 4 x magnification during light microscopy, Olympus Provis AX70.
CT analysis of an OP and GJ pig lungs show the direct and indirect effects of aspiration over time (figure 2).
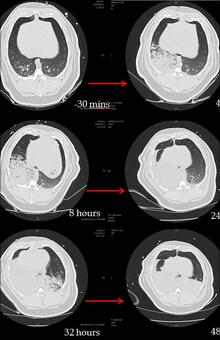
Figure 2: Time series CT lung scans taken from one pig at -30 minutes (before aspiration) to 48 hours after instillation of 0.5mls/kg mixture of OP and gastric juice in the right lung at time 0 hours. Over time, the right lung becomes more consolidated and at 32 hours the left lung also starts to show an indirect injury. At 48 hours bilateral posterior consolidation and fluid in the fissures are apparent.
Mixtures of OP and GJ and GJ alone when instilled into the right lung seem to create the worst lung injuries in comparison to solvent and GJ and control animals.
In order to test the validity of the minipig model, a pilot study in Sri Lanka was devised to observe signs of lung injury in human patients who had ingested OP insecticide with or without clinical evidence of pulmonary aspiration with some surgical controls.









